COVID-19 Surge, Idaho Activates Crisis Care
September 16, 2021
September 16, 2021
COVID-19 Surge, Idaho Activates Crisis Care
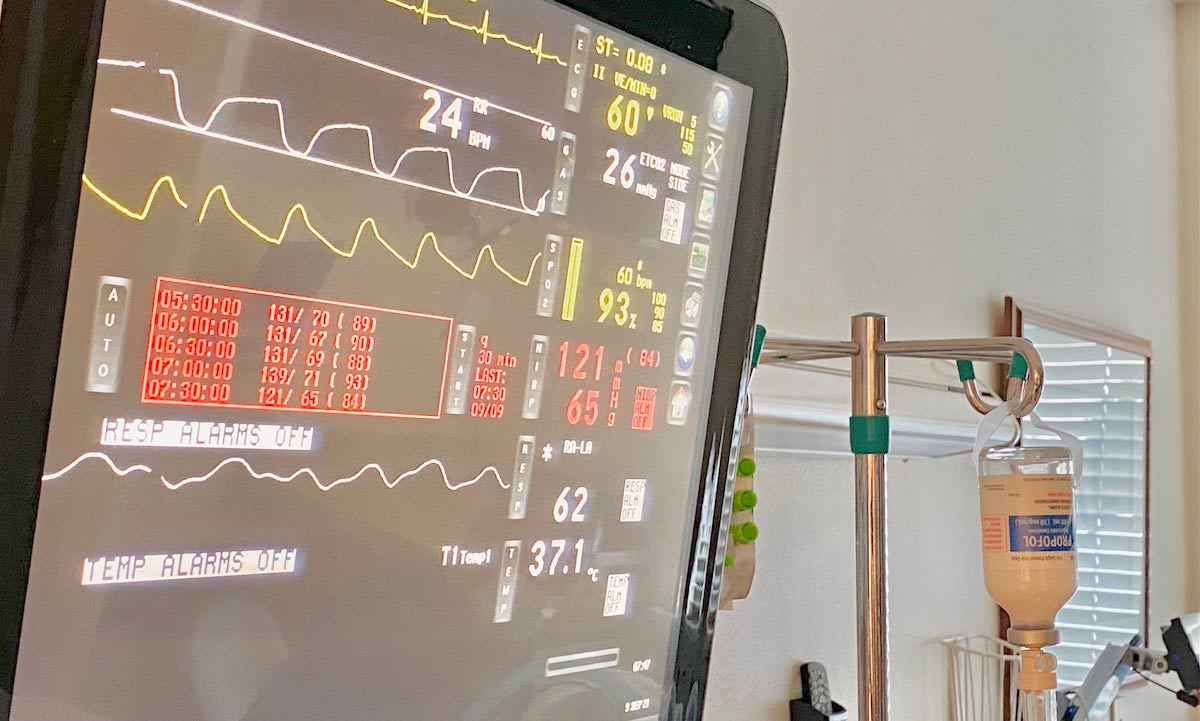
Current Visitor Restrictions at Madison Memorial:
 If you’ve already received your first dose of a two-dose COVID-19 vaccine, congratulations — you’re well on your way to being protected from the coronavirus. But to be fully immunized, it’s critical to get that second shot.
Across the country, some people are running into snafus as they try to get their second dose. Winter storms have shut down clinics in some areas, while others have closed because they temporarily ran out of vaccine. There are also scattered reports of scheduling glitches.
If you’ve had an appointment canceled, don’t wait for someone to call you — be proactive about rescheduling your second shot, advises William Schaffner, M.D., an infectious diseases specialist at Vanderbilt University Medical Center in Nashville, Tennessee, and medical director of the National Foundation for Infectious Diseases.
“We have told everyone these vaccines are 95 percent effective,” he says of the two-dose Moderna and Pfizer-BioNTech vaccines currently in use in the U.S. “But they’re only 95 percent effective if you indeed get that second dose.”
Here are a few more things to know about the second dose:
1. Your side effects will likely be stronger
Many people who had little to no reaction to the first vaccine dose are reporting that the second one packs a punch — surprising even those who study vaccines for a living.
Greg Poland, M.D., an infectious disease expert at the Mayo Clinic in Rochester, Minnesota, and director of Mayo’s vaccine research group, had only mild symptoms after his first dose. But the second one left him shaking — literally — with chills and a temperature of 101.
“I took one Tylenol and went to bed and woke up the next morning 90 percent improved, and by midday I was back to normal,” Poland says. “This is not an indication of something going wrong; it is an indication of a vigorous immune response.”
There is no live virus in the vaccine, so you can’t get COVID-19 from being vaccinated.
Participants in clinical trials of both vaccines had experiences similar to Poland’s. In Pfizer’s clinical trial, for instance, 31 percent of participants ages 18 to 55 reported a fever after the second dose, compared to only 8 percent after the first one. Fatigue, chills, headache and muscle/joint pain were also more common after the second injection for both vaccines.
The good news is, older adults were less likely to experience vaccine reactions, the data shows.
Among those age 55 and up in the Pfizer trial, 22 percent experienced fever after the second dose, and 3 percent had a temperature after the first dose.
Schaffner recommends not making any big plans for the day after your scheduled vaccine appointment.
2. You should avoid taking pain relievers before your shot
If you’ve been hearing stories about second-dose side effects, you may be tempted to take a pain reliever before your appointment.
That’s not a good idea, according to the U.S. Centers for Disease Control and Prevention (CDC), unless you’ve been advised to do so by your doctor. Pain relievers taken preemptively ahead of a shot could dampen the effectiveness of the vaccine, Poland and Schaffner say.
However, it’s OK to take acetaminophen (Tylenol) or a nonsteroidal anti-inflammatory drug like Advil
or Motrin after your vaccine to treat side effects such as pain, fever, chills or headache.
3. The timing between doses doesn’t need to be exact
The second dose of the Pfizer shot is supposed to be given 21 days after the first; for Moderna, the recommended interval between doses is 28 days.
However, if you can’t get an appointment on the exact day — or if you have to miss your scheduled appointment for some reason — the CDC does allow some wiggle room. Although the agency recommends trying to stick to the suggested interval, it says the second dose can be given up to six weeks after the first.
If your appointment is scheduled earlier than the recommended date, ask for a later appointment, Schaffner advises. “Your immune response will work perfectly well if you take more time,” he says. “But if you do it too early, the second dose may not invoke an optimal response.”
4. Your second dose should be from the same manufacturer as your first
Doctors are already hearing from patients asking if they can get their second dose from a different manufacturer, often because they realize the other type of vaccine is offered at a location that’s more convenient. But the CDC recommends against it: The Moderna and Pfizer vaccines “are not interchangeable with each other or with other COVID-19 vaccine products,” the CDC says. “The safety and efficacy of a mixed-product series have not been evaluated.”
The CDC does allow the mixing of Pfizer and Moderna shots in “exceptional situations,” such as when the vaccine used for someone’s first dose is no longer available due to a supply shortage, or if it’s unclear which vaccine they got for their first dose.
5. A rash at the injection site isn’t a reason to skip your second dose
If you experienced a rash at the injection site three to 10 days after getting your first shot, that doesn’t preclude you from getting your second shot, the CDC says, although it recommends getting it in the other arm.
A small number of people have developed such rashes, sometimes called “COVID arm,” after vaccination. Doctors say it’s likely a mild allergic reaction that can be treated with an over-the-counter antihistamine such as Benadryl.
In guidance released Feb. 10, the CDC says the reaction is not believed to represent a risk for a more severe allergic reaction when you get your second dose.
6. You should temporarily avoid all other vaccines
It might be time for your shingles or Tdap vaccine, but you should hold off if you are between COVID-19 vaccine doses. Because there’s no data on the safety and efficacy of COVID vaccines administered at the same time as other vaccines, the CDC recommends avoiding other immunizations in the two weeks before and after both doses. Holding off also helps prevent confusion about the cause of a reaction if you experience one.
The CDC does allow exceptions in circumstances where avoiding the vaccine would put you at risk, such as a tetanus shot after a wound or a hepatitis shot during an outbreak.
7. Full immunity is not immediate
It takes two weeks after your second dose for your body to build full protection to the virus. After that, you should have almost zero chance of developing severe disease if you are exposed to someone with COVID-19, Schaffner says. The CDC also says you no longer have to quarantine if you’re exposed to someone with COVID-19 — as long as you meet these criteria: you don’t have symptoms and it hasn’t been more than three months since your second vaccine dose.
One possible exception is immunocompromised people, Schaffner says. They will get some level of immunity, he says, “but they may not reach the 95 percent because their immune system is already somewhat compromised, no matter how strong these vaccines are.”
8. You still need to wear a mask
Experts are divided about whether it’s OK to hug your grandchild or gather socially with other vaccinated people after you’re fully immunized.
But they agree you should continue to wear a mask and practice social distancing in public. For one thing, there’s a small chance you could get sick even after you’ve been vaccinated.
In addition, it’s possible that you could still carry the virus and silently transmit it to others who haven’t been vaccinated, even if you don’t develop symptoms.
And there’s one more reason. Until the country reaches herd immunity — the point when a significant portion of the population becomes immune to a disease — it’s important for everyone to wear a mask to stop the spread of the virus, Schaffner says. “If we have some people walking around maskless and others not, people left and right are just going to discard their masks,” he says. “We are not ready yet for that for society. Let’s all stick to masks a little longer until we get the all clear.”
Michelle Crouch is a contributing writer who has covered health and personal finance for some of the nation’s top consumer publications. Her work has appeared in Reader’s Digest, Real Simple, Prevention, The Washington Post and The New York Times.
If you’ve already received your first dose of a two-dose COVID-19 vaccine, congratulations — you’re well on your way to being protected from the coronavirus. But to be fully immunized, it’s critical to get that second shot.
Across the country, some people are running into snafus as they try to get their second dose. Winter storms have shut down clinics in some areas, while others have closed because they temporarily ran out of vaccine. There are also scattered reports of scheduling glitches.
If you’ve had an appointment canceled, don’t wait for someone to call you — be proactive about rescheduling your second shot, advises William Schaffner, M.D., an infectious diseases specialist at Vanderbilt University Medical Center in Nashville, Tennessee, and medical director of the National Foundation for Infectious Diseases.
“We have told everyone these vaccines are 95 percent effective,” he says of the two-dose Moderna and Pfizer-BioNTech vaccines currently in use in the U.S. “But they’re only 95 percent effective if you indeed get that second dose.”
Here are a few more things to know about the second dose:
1. Your side effects will likely be stronger
Many people who had little to no reaction to the first vaccine dose are reporting that the second one packs a punch — surprising even those who study vaccines for a living.
Greg Poland, M.D., an infectious disease expert at the Mayo Clinic in Rochester, Minnesota, and director of Mayo’s vaccine research group, had only mild symptoms after his first dose. But the second one left him shaking — literally — with chills and a temperature of 101.
“I took one Tylenol and went to bed and woke up the next morning 90 percent improved, and by midday I was back to normal,” Poland says. “This is not an indication of something going wrong; it is an indication of a vigorous immune response.”
There is no live virus in the vaccine, so you can’t get COVID-19 from being vaccinated.
Participants in clinical trials of both vaccines had experiences similar to Poland’s. In Pfizer’s clinical trial, for instance, 31 percent of participants ages 18 to 55 reported a fever after the second dose, compared to only 8 percent after the first one. Fatigue, chills, headache and muscle/joint pain were also more common after the second injection for both vaccines.
The good news is, older adults were less likely to experience vaccine reactions, the data shows.
Among those age 55 and up in the Pfizer trial, 22 percent experienced fever after the second dose, and 3 percent had a temperature after the first dose.
Schaffner recommends not making any big plans for the day after your scheduled vaccine appointment.
2. You should avoid taking pain relievers before your shot
If you’ve been hearing stories about second-dose side effects, you may be tempted to take a pain reliever before your appointment.
That’s not a good idea, according to the U.S. Centers for Disease Control and Prevention (CDC), unless you’ve been advised to do so by your doctor. Pain relievers taken preemptively ahead of a shot could dampen the effectiveness of the vaccine, Poland and Schaffner say.
However, it’s OK to take acetaminophen (Tylenol) or a nonsteroidal anti-inflammatory drug like Advil
or Motrin after your vaccine to treat side effects such as pain, fever, chills or headache.
3. The timing between doses doesn’t need to be exact
The second dose of the Pfizer shot is supposed to be given 21 days after the first; for Moderna, the recommended interval between doses is 28 days.
However, if you can’t get an appointment on the exact day — or if you have to miss your scheduled appointment for some reason — the CDC does allow some wiggle room. Although the agency recommends trying to stick to the suggested interval, it says the second dose can be given up to six weeks after the first.
If your appointment is scheduled earlier than the recommended date, ask for a later appointment, Schaffner advises. “Your immune response will work perfectly well if you take more time,” he says. “But if you do it too early, the second dose may not invoke an optimal response.”
4. Your second dose should be from the same manufacturer as your first
Doctors are already hearing from patients asking if they can get their second dose from a different manufacturer, often because they realize the other type of vaccine is offered at a location that’s more convenient. But the CDC recommends against it: The Moderna and Pfizer vaccines “are not interchangeable with each other or with other COVID-19 vaccine products,” the CDC says. “The safety and efficacy of a mixed-product series have not been evaluated.”
The CDC does allow the mixing of Pfizer and Moderna shots in “exceptional situations,” such as when the vaccine used for someone’s first dose is no longer available due to a supply shortage, or if it’s unclear which vaccine they got for their first dose.
5. A rash at the injection site isn’t a reason to skip your second dose
If you experienced a rash at the injection site three to 10 days after getting your first shot, that doesn’t preclude you from getting your second shot, the CDC says, although it recommends getting it in the other arm.
A small number of people have developed such rashes, sometimes called “COVID arm,” after vaccination. Doctors say it’s likely a mild allergic reaction that can be treated with an over-the-counter antihistamine such as Benadryl.
In guidance released Feb. 10, the CDC says the reaction is not believed to represent a risk for a more severe allergic reaction when you get your second dose.
6. You should temporarily avoid all other vaccines
It might be time for your shingles or Tdap vaccine, but you should hold off if you are between COVID-19 vaccine doses. Because there’s no data on the safety and efficacy of COVID vaccines administered at the same time as other vaccines, the CDC recommends avoiding other immunizations in the two weeks before and after both doses. Holding off also helps prevent confusion about the cause of a reaction if you experience one.
The CDC does allow exceptions in circumstances where avoiding the vaccine would put you at risk, such as a tetanus shot after a wound or a hepatitis shot during an outbreak.
7. Full immunity is not immediate
It takes two weeks after your second dose for your body to build full protection to the virus. After that, you should have almost zero chance of developing severe disease if you are exposed to someone with COVID-19, Schaffner says. The CDC also says you no longer have to quarantine if you’re exposed to someone with COVID-19 — as long as you meet these criteria: you don’t have symptoms and it hasn’t been more than three months since your second vaccine dose.
One possible exception is immunocompromised people, Schaffner says. They will get some level of immunity, he says, “but they may not reach the 95 percent because their immune system is already somewhat compromised, no matter how strong these vaccines are.”
8. You still need to wear a mask
Experts are divided about whether it’s OK to hug your grandchild or gather socially with other vaccinated people after you’re fully immunized.
But they agree you should continue to wear a mask and practice social distancing in public. For one thing, there’s a small chance you could get sick even after you’ve been vaccinated.
In addition, it’s possible that you could still carry the virus and silently transmit it to others who haven’t been vaccinated, even if you don’t develop symptoms.
And there’s one more reason. Until the country reaches herd immunity — the point when a significant portion of the population becomes immune to a disease — it’s important for everyone to wear a mask to stop the spread of the virus, Schaffner says. “If we have some people walking around maskless and others not, people left and right are just going to discard their masks,” he says. “We are not ready yet for that for society. Let’s all stick to masks a little longer until we get the all clear.”
Michelle Crouch is a contributing writer who has covered health and personal finance for some of the nation’s top consumer publications. Her work has appeared in Reader’s Digest, Real Simple, Prevention, The Washington Post and The New York Times.
-
- There can only be one (1) designated visitor per patient, for the length of the patient’s stay (i.e., not one visitor at a time; only one visitor, who may come and go as needed, but is always the same person)
- No one under 18 may enter the building unless that person is a patient
- The Paragon Café continues to be closed to the general public
- Parents of pediatric patients may request permission to both (jointly or separately) visit their child
FAQ (Frequently Asked Questions)
What are Crisis Standards of Care? Crisis standards of care (CSC) are guidelines that help healthcare providers and systems decide how to deliver the best care possible under the extraordinary circumstances of an overwhelming disaster or public health emergency. The guidelines may be used when there are not enough healthcare resources to provide the usual standard of care to people who need it. The goal of crisis standards of care is to extend care to as many patients as possible and save as many lives as possible. How will hospital care be different than at other times when there is not a crisis? When crisis standards of care are in effect, people who need medical care may experience care that is different from what they expect. For example, patients admitted to the hospital may find that hospital beds are not available or are in repurposed rooms (such as a conference room) or that needed equipment is not available. Who decides when a hospital needs to activate Crisis Standards of Care (CSC)? The director of the Department of Health and Welfare or his delegate will declare the activation of crisis standards of care after careful consideration of the resource shortages that necessitate crisis standards of care and the measures that have been taken to address these shortages. Can hospitals share resources instead of implementing crisis standards of care? Yes. Before crisis standards of care are implemented, every effort is made to secure resources from local, regional, and federal sources. Crisis standards of care will ONLY be implemented if sufficient resources cannot be obtained quickly enough to provide adequate care for patients. How long will Crisis Standards of Care (CSC) be in place for select Idaho hospitals? The crisis standards of care will remain in effect until there are sufficient resources to provide the usual standard of care to all patients. If crisis standards of care are implemented during the COVID-19 pandemic, will all medical care be affected, or just COVID-19-related care? If crisis standards of care are implemented during the COVID-19 pandemic, all types of medical care may be affected. If, for example, a patient needs ICU level care for the treatment of a severe infection or a traumatic accident, and there are not enough ICU beds available to treat all the patients who need one, that patient would enter a triage algorithm just like patients with COVID-19 who need an ICU bed. Has Idaho ever had to utilize crisis standards of care? The first implementation of the Idaho Crisis Standards of Care Plan went into effect for the first time in September of 2021. For the Idaho panhandle the process to initiate crisis standards of care began when resources were limited to the point of affecting medical care. The director of DHW convened the Crisis Standards of Care Activation Advisory Committee on Sept. 6, 2021, to review all the measures that were taken to address the staffing and bed shortages. The committee determined that the ability of northern Idaho hospitals and healthcare systems to deliver the usual standard of care has been severely affected by the staffing shortages, and all contingency measures to address these shortages had been exhausted. The committee recommended to the director that crisis standards of care be activated. Director Jeppesen issued his decision on Sept. 6, 2021, under the authority vested in him through the temporary rule How can I get up-to-date information about the status of the Idaho health care system? healthandwelfare.idaho.gov. COVID-19 Cases Surge in Eastern Idaho as Hospitals Request SupportSeptember 8, 2021
In communities across Idaho, we have celebrated the impact of our brave, dedicated healthcare workers as they fight against COVID-19. This pandemic, now in its 18th month, continues to take a relentless toll on the region’s caregivers. They have worked tirelessly and devoted themselves to caring for patients; now they need your help. Hospitals throughout the region are currently experiencing COVID-19 hospitalization rates exceeding those of the peak surge in December 2020. While our facilities remain open and are capable of caring for patients in need, the current COVID-19 crisis puts a significant strain on hospital resources, including staff and bed availability. This increase has caused local ICU and inpatient hospital floors to reach capacity on a regular basis. Our hospitals have experienced a continual flow of patients. To manage resources, hospital leaders at each facility monitor staffing and bed availability continuously throughout the day. Additionally, we collectively assess resources across hospitals to help meet the needs of our region. In late spring, hospitals in our region had single-digit COVID-19 admissions. We were hopeful that the pandemic was ending. Unfortunately, this is not the case. Four of the largest hospitals in the region (EIRMC, Portneuf Medical Center, Idaho Falls Community Hospital, and Madison Memorial Hospital) collectively admitted a total of 74 inpatients with COVID illness in the month of July. That number soared to 157 in the month of August, representing a 112% increase. Patients hospitalized with COVID-19 illness are overwhelmingly unvaccinated. Hospitals in our region are experiencing similar COVID-19 trends to those reported in other states and around the nation. We are also seeing younger and generally healthier people needing hospital care after contracting the virus. We strongly encourage people in our area to get the vaccine and follow the CDC’s recommendations for preventing the spread of the virus, including guidance around mask usage, social distancing, and frequent hand washing. The vaccine has been shown to be safe and effective, with 370 million doses administered across the U.S., as of September 1, 2021. As of this date, nearly 740,000 Idahoans are fully vaccinated. This leaves roughly 60% of Gem State residents unprotected. The Food & Drug Administration has granted full approval of the Pfizer COVID-19 vaccine for individuals 16 years of age and older. The vaccine is also available for those as young as 12 under Emergency Use Authorization (EUA). The Moderna and Johnson & Johnson vaccines also remain in widespread use under EUA while seeking full approval. The choices Idahoans make have a direct impact on whether hospitals have the human resources necessary to care for our community. We are imploring our community to get the COVID-19 vaccine. Protect yourself, protect our children, and protect the vulnerable members of our community unable to receive the vaccine. Do your part to make sure we can protect our precious hospital resources and care for those who need us most. These hospitals in south and southeastern Idaho stand united in this joint statement.- Eastern Idaho Regional Medical Center
- Portneuf Medical Center
- Madison Memorial Hospital
- Idaho Falls Community Hospital
- Mountain View Hospital
- Bingham Memorial Hospital
- Steele Memorial Medical Center
- Teton Valley Hospital
- Bear Lake Memorial Hospital
- Minidoka Memorial Hospital
- Franklin County Medical Center
- Nell J. Redfield Memorial Hospital
- Power County Hospital District
- Intermountain Cassia Regional Hospital
Johnson & Johnson’s Janssen Vaccine
Possible Side Effects
In the arm where you got the shot:- Pain
- Redness
- Swelling
- Tiredness
- Headache
- Muscle pain
- Chills
- Fever
- Nausea
Safety
- In clinical trials, side effects were common within 7 days of getting vaccinated but were mostly mild to moderate.
- Side effects were more common in people 18–59 years old compared to people 60 years and older.
- CDC will continue to provide updates as we learn more about the safety of the J&J/Janssen vaccine in real-world conditions.
Pfizer Vaccine
Possible Side Effects
In the arm where you got the shot:- Pain
- Redness
- Swelling
- Tiredness
- Headache
- Muscle pain
- Chills
- Fever
- Nausea
Safety
-
- In clinical trials, reactogenicity symptoms (side effects that happen within 7 days of getting vaccinated) were common but were mostly mild to moderate.
- Side effects (such as fever, chills, tiredness, and headache) throughout the body were more common after the second dose of the vaccine.
- Most side effects were mild to moderate. However, a small number of people had severe side effects—defined as side effects affecting a person’s ability to do daily activities.
- Although few people in the clinical trials went to the hospital or died, data suggest that people who got the Pfizer-BioNTech vaccine were less likely to have these more serious outcomes compared to people who got the saline placebo.
- CDC will continue to provide updates as we learn more about the safety of the Pfizer-BioNTech vaccine in real-world conditions.
Moderna Vaccine
Possible Side Effects
In the arm where you got the shot:- Pain
- Redness
- Swelling
- Tiredness
- Headache
- Muscle pain
- Chills
- Fever
- Nausea
Safety
- In clinical trials, reactogenicity symptoms (side effects that happen within 7 days of getting vaccinated) were common but were mostly mild to moderate.
- Side effects (such as fever, chills, tiredness, and headache) throughout the body were more common after the second dose of the vaccine.
- Most side effects were mild to moderate. However, a small number of people had severe side effects that affected their ability to do daily activities.
- CDC will continue to provide updates as we learn more about the safety of the Moderna vaccine in real-world conditions.
Vaccination Dates and Locations
8 Things to Know Before Your Second COVID-19 Vaccine
Understand the do’s and don’ts of the two-dose coronavirus vaccination regimen by Michelle Crouch, AARP, February 17, 2021 If you’ve already received your first dose of a two-dose COVID-19 vaccine, congratulations — you’re well on your way to being protected from the coronavirus. But to be fully immunized, it’s critical to get that second shot.
Across the country, some people are running into snafus as they try to get their second dose. Winter storms have shut down clinics in some areas, while others have closed because they temporarily ran out of vaccine. There are also scattered reports of scheduling glitches.
If you’ve had an appointment canceled, don’t wait for someone to call you — be proactive about rescheduling your second shot, advises William Schaffner, M.D., an infectious diseases specialist at Vanderbilt University Medical Center in Nashville, Tennessee, and medical director of the National Foundation for Infectious Diseases.
“We have told everyone these vaccines are 95 percent effective,” he says of the two-dose Moderna and Pfizer-BioNTech vaccines currently in use in the U.S. “But they’re only 95 percent effective if you indeed get that second dose.”
Here are a few more things to know about the second dose:
1. Your side effects will likely be stronger
Many people who had little to no reaction to the first vaccine dose are reporting that the second one packs a punch — surprising even those who study vaccines for a living.
Greg Poland, M.D., an infectious disease expert at the Mayo Clinic in Rochester, Minnesota, and director of Mayo’s vaccine research group, had only mild symptoms after his first dose. But the second one left him shaking — literally — with chills and a temperature of 101.
“I took one Tylenol and went to bed and woke up the next morning 90 percent improved, and by midday I was back to normal,” Poland says. “This is not an indication of something going wrong; it is an indication of a vigorous immune response.”
There is no live virus in the vaccine, so you can’t get COVID-19 from being vaccinated.
Participants in clinical trials of both vaccines had experiences similar to Poland’s. In Pfizer’s clinical trial, for instance, 31 percent of participants ages 18 to 55 reported a fever after the second dose, compared to only 8 percent after the first one. Fatigue, chills, headache and muscle/joint pain were also more common after the second injection for both vaccines.
The good news is, older adults were less likely to experience vaccine reactions, the data shows.
Among those age 55 and up in the Pfizer trial, 22 percent experienced fever after the second dose, and 3 percent had a temperature after the first dose.
Schaffner recommends not making any big plans for the day after your scheduled vaccine appointment.
2. You should avoid taking pain relievers before your shot
If you’ve been hearing stories about second-dose side effects, you may be tempted to take a pain reliever before your appointment.
That’s not a good idea, according to the U.S. Centers for Disease Control and Prevention (CDC), unless you’ve been advised to do so by your doctor. Pain relievers taken preemptively ahead of a shot could dampen the effectiveness of the vaccine, Poland and Schaffner say.
However, it’s OK to take acetaminophen (Tylenol) or a nonsteroidal anti-inflammatory drug like Advil
or Motrin after your vaccine to treat side effects such as pain, fever, chills or headache.
3. The timing between doses doesn’t need to be exact
The second dose of the Pfizer shot is supposed to be given 21 days after the first; for Moderna, the recommended interval between doses is 28 days.
However, if you can’t get an appointment on the exact day — or if you have to miss your scheduled appointment for some reason — the CDC does allow some wiggle room. Although the agency recommends trying to stick to the suggested interval, it says the second dose can be given up to six weeks after the first.
If your appointment is scheduled earlier than the recommended date, ask for a later appointment, Schaffner advises. “Your immune response will work perfectly well if you take more time,” he says. “But if you do it too early, the second dose may not invoke an optimal response.”
4. Your second dose should be from the same manufacturer as your first
Doctors are already hearing from patients asking if they can get their second dose from a different manufacturer, often because they realize the other type of vaccine is offered at a location that’s more convenient. But the CDC recommends against it: The Moderna and Pfizer vaccines “are not interchangeable with each other or with other COVID-19 vaccine products,” the CDC says. “The safety and efficacy of a mixed-product series have not been evaluated.”
The CDC does allow the mixing of Pfizer and Moderna shots in “exceptional situations,” such as when the vaccine used for someone’s first dose is no longer available due to a supply shortage, or if it’s unclear which vaccine they got for their first dose.
5. A rash at the injection site isn’t a reason to skip your second dose
If you experienced a rash at the injection site three to 10 days after getting your first shot, that doesn’t preclude you from getting your second shot, the CDC says, although it recommends getting it in the other arm.
A small number of people have developed such rashes, sometimes called “COVID arm,” after vaccination. Doctors say it’s likely a mild allergic reaction that can be treated with an over-the-counter antihistamine such as Benadryl.
In guidance released Feb. 10, the CDC says the reaction is not believed to represent a risk for a more severe allergic reaction when you get your second dose.
6. You should temporarily avoid all other vaccines
It might be time for your shingles or Tdap vaccine, but you should hold off if you are between COVID-19 vaccine doses. Because there’s no data on the safety and efficacy of COVID vaccines administered at the same time as other vaccines, the CDC recommends avoiding other immunizations in the two weeks before and after both doses. Holding off also helps prevent confusion about the cause of a reaction if you experience one.
The CDC does allow exceptions in circumstances where avoiding the vaccine would put you at risk, such as a tetanus shot after a wound or a hepatitis shot during an outbreak.
7. Full immunity is not immediate
It takes two weeks after your second dose for your body to build full protection to the virus. After that, you should have almost zero chance of developing severe disease if you are exposed to someone with COVID-19, Schaffner says. The CDC also says you no longer have to quarantine if you’re exposed to someone with COVID-19 — as long as you meet these criteria: you don’t have symptoms and it hasn’t been more than three months since your second vaccine dose.
One possible exception is immunocompromised people, Schaffner says. They will get some level of immunity, he says, “but they may not reach the 95 percent because their immune system is already somewhat compromised, no matter how strong these vaccines are.”
8. You still need to wear a mask
Experts are divided about whether it’s OK to hug your grandchild or gather socially with other vaccinated people after you’re fully immunized.
But they agree you should continue to wear a mask and practice social distancing in public. For one thing, there’s a small chance you could get sick even after you’ve been vaccinated.
In addition, it’s possible that you could still carry the virus and silently transmit it to others who haven’t been vaccinated, even if you don’t develop symptoms.
And there’s one more reason. Until the country reaches herd immunity — the point when a significant portion of the population becomes immune to a disease — it’s important for everyone to wear a mask to stop the spread of the virus, Schaffner says. “If we have some people walking around maskless and others not, people left and right are just going to discard their masks,” he says. “We are not ready yet for that for society. Let’s all stick to masks a little longer until we get the all clear.”
Michelle Crouch is a contributing writer who has covered health and personal finance for some of the nation’s top consumer publications. Her work has appeared in Reader’s Digest, Real Simple, Prevention, The Washington Post and The New York Times.
If you’ve already received your first dose of a two-dose COVID-19 vaccine, congratulations — you’re well on your way to being protected from the coronavirus. But to be fully immunized, it’s critical to get that second shot.
Across the country, some people are running into snafus as they try to get their second dose. Winter storms have shut down clinics in some areas, while others have closed because they temporarily ran out of vaccine. There are also scattered reports of scheduling glitches.
If you’ve had an appointment canceled, don’t wait for someone to call you — be proactive about rescheduling your second shot, advises William Schaffner, M.D., an infectious diseases specialist at Vanderbilt University Medical Center in Nashville, Tennessee, and medical director of the National Foundation for Infectious Diseases.
“We have told everyone these vaccines are 95 percent effective,” he says of the two-dose Moderna and Pfizer-BioNTech vaccines currently in use in the U.S. “But they’re only 95 percent effective if you indeed get that second dose.”
Here are a few more things to know about the second dose:
1. Your side effects will likely be stronger
Many people who had little to no reaction to the first vaccine dose are reporting that the second one packs a punch — surprising even those who study vaccines for a living.
Greg Poland, M.D., an infectious disease expert at the Mayo Clinic in Rochester, Minnesota, and director of Mayo’s vaccine research group, had only mild symptoms after his first dose. But the second one left him shaking — literally — with chills and a temperature of 101.
“I took one Tylenol and went to bed and woke up the next morning 90 percent improved, and by midday I was back to normal,” Poland says. “This is not an indication of something going wrong; it is an indication of a vigorous immune response.”
There is no live virus in the vaccine, so you can’t get COVID-19 from being vaccinated.
Participants in clinical trials of both vaccines had experiences similar to Poland’s. In Pfizer’s clinical trial, for instance, 31 percent of participants ages 18 to 55 reported a fever after the second dose, compared to only 8 percent after the first one. Fatigue, chills, headache and muscle/joint pain were also more common after the second injection for both vaccines.
The good news is, older adults were less likely to experience vaccine reactions, the data shows.
Among those age 55 and up in the Pfizer trial, 22 percent experienced fever after the second dose, and 3 percent had a temperature after the first dose.
Schaffner recommends not making any big plans for the day after your scheduled vaccine appointment.
2. You should avoid taking pain relievers before your shot
If you’ve been hearing stories about second-dose side effects, you may be tempted to take a pain reliever before your appointment.
That’s not a good idea, according to the U.S. Centers for Disease Control and Prevention (CDC), unless you’ve been advised to do so by your doctor. Pain relievers taken preemptively ahead of a shot could dampen the effectiveness of the vaccine, Poland and Schaffner say.
However, it’s OK to take acetaminophen (Tylenol) or a nonsteroidal anti-inflammatory drug like Advil
or Motrin after your vaccine to treat side effects such as pain, fever, chills or headache.
3. The timing between doses doesn’t need to be exact
The second dose of the Pfizer shot is supposed to be given 21 days after the first; for Moderna, the recommended interval between doses is 28 days.
However, if you can’t get an appointment on the exact day — or if you have to miss your scheduled appointment for some reason — the CDC does allow some wiggle room. Although the agency recommends trying to stick to the suggested interval, it says the second dose can be given up to six weeks after the first.
If your appointment is scheduled earlier than the recommended date, ask for a later appointment, Schaffner advises. “Your immune response will work perfectly well if you take more time,” he says. “But if you do it too early, the second dose may not invoke an optimal response.”
4. Your second dose should be from the same manufacturer as your first
Doctors are already hearing from patients asking if they can get their second dose from a different manufacturer, often because they realize the other type of vaccine is offered at a location that’s more convenient. But the CDC recommends against it: The Moderna and Pfizer vaccines “are not interchangeable with each other or with other COVID-19 vaccine products,” the CDC says. “The safety and efficacy of a mixed-product series have not been evaluated.”
The CDC does allow the mixing of Pfizer and Moderna shots in “exceptional situations,” such as when the vaccine used for someone’s first dose is no longer available due to a supply shortage, or if it’s unclear which vaccine they got for their first dose.
5. A rash at the injection site isn’t a reason to skip your second dose
If you experienced a rash at the injection site three to 10 days after getting your first shot, that doesn’t preclude you from getting your second shot, the CDC says, although it recommends getting it in the other arm.
A small number of people have developed such rashes, sometimes called “COVID arm,” after vaccination. Doctors say it’s likely a mild allergic reaction that can be treated with an over-the-counter antihistamine such as Benadryl.
In guidance released Feb. 10, the CDC says the reaction is not believed to represent a risk for a more severe allergic reaction when you get your second dose.
6. You should temporarily avoid all other vaccines
It might be time for your shingles or Tdap vaccine, but you should hold off if you are between COVID-19 vaccine doses. Because there’s no data on the safety and efficacy of COVID vaccines administered at the same time as other vaccines, the CDC recommends avoiding other immunizations in the two weeks before and after both doses. Holding off also helps prevent confusion about the cause of a reaction if you experience one.
The CDC does allow exceptions in circumstances where avoiding the vaccine would put you at risk, such as a tetanus shot after a wound or a hepatitis shot during an outbreak.
7. Full immunity is not immediate
It takes two weeks after your second dose for your body to build full protection to the virus. After that, you should have almost zero chance of developing severe disease if you are exposed to someone with COVID-19, Schaffner says. The CDC also says you no longer have to quarantine if you’re exposed to someone with COVID-19 — as long as you meet these criteria: you don’t have symptoms and it hasn’t been more than three months since your second vaccine dose.
One possible exception is immunocompromised people, Schaffner says. They will get some level of immunity, he says, “but they may not reach the 95 percent because their immune system is already somewhat compromised, no matter how strong these vaccines are.”
8. You still need to wear a mask
Experts are divided about whether it’s OK to hug your grandchild or gather socially with other vaccinated people after you’re fully immunized.
But they agree you should continue to wear a mask and practice social distancing in public. For one thing, there’s a small chance you could get sick even after you’ve been vaccinated.
In addition, it’s possible that you could still carry the virus and silently transmit it to others who haven’t been vaccinated, even if you don’t develop symptoms.
And there’s one more reason. Until the country reaches herd immunity — the point when a significant portion of the population becomes immune to a disease — it’s important for everyone to wear a mask to stop the spread of the virus, Schaffner says. “If we have some people walking around maskless and others not, people left and right are just going to discard their masks,” he says. “We are not ready yet for that for society. Let’s all stick to masks a little longer until we get the all clear.”
Michelle Crouch is a contributing writer who has covered health and personal finance for some of the nation’s top consumer publications. Her work has appeared in Reader’s Digest, Real Simple, Prevention, The Washington Post and The New York Times.
First COVID-19 Vaccination in Idaho received at Madison Memorial Dec. 14th, 2020
A number of Madison Memorial employees who work on the front lines of the pandemic are excited to have been among the first in Idaho to receive the newly approved COVID-19 vaccine. Dr. Steven Lofgran, MD, who participated in the Pfizer trial, and Dr. Hancock who have both now received the vaccine answer some of the most commonly asked questions regarding the vaccine, below. A majority of the healthcare workers at Madison Memorial Hospital have now received the vaccine. The vaccine consists of 2-shot series with a few weeks between each shot. Your Top 5 COVID Vaccine Questions, Answered 1. Will the vaccine give me COVID? No. That is impossible. Other types of vaccines sometimes carry weakened viruses; this one does not. It contains ZERO coronavirus, living or dead. Which means, there’s zero chance that it can give you COVID-19. 2. Will I have an allergic reaction? You might. That is a risk with any vaccine, but it will most likely be mild. Roughly half of all MMH employees have now received the COVID vaccine. About one in ten of them are reporting mild fever and bodyaches, lasting for about a day. Almost everyone, however, reports a bit of stiffness or swelling in the shoulder for a few days, but very little else. Even with millions of doses now administered, there have been very, very few serious reactions to the vaccine. 3. Because it was rushed through, was the vaccine not adequately tested? One reason that the approved vaccines were able to be approved so quickly was because they demonstrated a very high success rate, and a very low complication rate. The approval would actually take longer to receive if either of these things was more questionable. 4. Will vaccination protect me against new mutations of the virus? Yes, we think so. The mRNA vaccine targets a spike protein, rather than a single strain of the virus, leaving the body to manufacture its own broad range of effective antibodies. It’s expected that these antibodies will continue to work on future mutations, as well as on the currently known versions of the virus. 5. Will the vaccine make me infertile? No. Almost 3 million people in the US have now received the vaccine, with no known impact on reproductive ability. The infertility rumor began with a now-debunked Facebook post. There have been other rumors circulating on social media, and there will likely be more. We will stay abreast of the actual facts regarding complications and effectiveness of vaccines as they continue to be administered to large populations.- How quickly will the COVID-19 pandemic end?
- Who will get the vaccine first?
- I already had COVID-19. Do I still need the vaccine?
- How long will my immunity last?
- How strong will my immunity be?
- How many Covid-19 vaccine shots will I need?
- How many people in the Pfizer trial had side effects?
- What side effects can I expect?
- What about long-term effects?
- How exactly does the vaccine work?
- After my vaccination, can I stop wearing my mask?
- Does the vaccine contain a live virus?
- Is it safe for elderly people?
- Can I get the vaccine if I currently have COVID-19?
- What if I’m immuno-compromised?
- Does my immunity not begin until I receive the second shot?
- Is it safe for pregnant women?
- Is there any chance that the vaccine might actually give me COVID?
- What’s the possibility that the vaccine could cause autism?
- Are you yourself going to get the vaccine shot?
